Navigating Stem Cell Therapies
Please NOTE: This page is currently being edited. We apologize for the inconvenience.
When a person receives a diagnosis such as Parkinson’s disease or any other condition or disease for which there is no treatment or cure, their world is turned upside down. They walk out of their healthcare provider’s office reeling. Once home, they search the internet looking for information, alternative treatment options, and, simply looking for hope. Many patients search for regenerative medicine (stem cell) based therapies only to find they are left with more unanswered questions.
Navigating stem cell information and therapies on the internet is difficult at best. Today, there is a plethora of information available on the internet. It can be frustrating sorting through search results. How do you know what information is reliable and what is not?
The FDA warns against unapproved stem cell therapies (or “clinical trials”) provided by “bad actor” stem cell clinics (or businesses) offering promises of “cures” for those with unmet medical needs. There are approximately 1,000 bad actor stem cell clinics in the U.S. today promising relief for chronic pain, arthritis, joint damage, Alzheimer’s disease, Parkinson’s disease and other disorders without a cure or treatment. How can you separate hype from hope?
CLICK HERE to jump to FDA section.
A part of Summit for Stem Cell Foundation’s (Summit’s) mission is to empower patients, physicians and caregivers with information to elevate their understanding in order to manage their healthcare. This page is dedicated to exactly that…
- STEM CELL OVERVIEW
Many patients are unaware there are many different types of stem cells (e.g. bone marrow stem cells, embryonic stem cells, adult stem cells, umbilical cord stem cells, mesenchymal stem cells, adipose stem cells, induced pluripotent stem cells, etc.). What differentiates one stem cell type from another is how many cell types they can become. Based on this feature we can categorize all stem cell types into two general categories: multipotent or pluripotent.
- Adult or Multipotent Stem Cells can become two or more cell types in the body but NOT ALL cell types in the body (including fetal stem cells).
- Pluripotent Stem Cells can become ALL types of cells in the body.
Pluripotent Stem Cells
- An undifferentiated cell that is capable of finite self-renewal and can differentiate into every cell type in the body. Types: Embryonic stem cells and induced pluripotent stem cells.
- Pluripotent stem cells are NOT found in adult or fetal tissues or placenta/amnion.
- Pluripotent stem cells can differentiate into every cell type in the body.
Adult or Mulitpotent Stem Cells (including Fetal Stem Cells)
- An undifferentiated cell in the body that is capable of indefinite self-renewal and can differentiate into at least two cell types but not every cell type in the body. Fetal cells are considered to be adult stem cells. Types: Hematopoietic (blood) stem cells, mesenchymal (connective tissue) stem cells, adipose stem cells, fetal neural stem cells.
- Adult or Multipotent Stem Cells (including fetal stem cells) cannot become every cell type in the body.
- For more detailed information about stem cells. Click here: Stem Cell
WHAT IS STEM CELL DIFFERENTIATION?
Stem Cell differentiation is the process by which a more specialized cell is formed from a stem cell. These changes are largely due to highly controlled modifications in gene expression to become a more specific or specialized type of cell.
Please note: Adult or Multipotent Stem Cells have differentiation limitations.
TYPES OF STEM CELLS AND THEIR CHARACTERISTICS
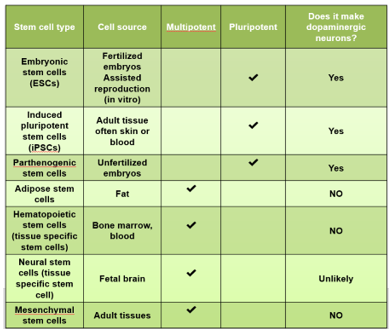
WHY STEM CELL THERAPY?
Based on years of experience, Summit believes cell-based therapies have the potential to fully integrate and thereby heal and, someday, cure the human nervous system. Summit supports all types of evidence-based research investigating the causes, prevention, and treatment of Parkinson’s disease and neurodegenerative conditions. The research Summit is focused on must be either within the field of regenerative medicine or has the potential to affect/influence the field of regenerative medicine.
Summit’s flagship research project, cell-based dopamine neuron replacement therapy, is aimed at providing lasting symptomatic relief for the physical symptoms of Parkinson’s disease. Summit provided support and funding for the research project guided by Dr. Jeanne Loring. In an effort to hasten the journey from bench to bedside, the program was eventually transferred to a newly formed private business, Aspen Neuroscience. Since then, the project has progressed from a research phase to a preclinical phase. The company plans to launch the first clinical trial in 2022. For more information about this project, CLICK HERE
QUESTIONS YOU SHOULD ASK:
Before participation in any therapy, treatment or clinical trial using either stem cells or is sourced from stem-cells, Summit’s scientific advisors suggest that the following 7 questions be answered:
- What is being transplanted, and how do the transplanted cells act to reduce symptoms?
2a. If you are in the United States, is the study/therapy/treatment FDA approved? https://clinicaltrials.gov/ct2/home
OR 2b. If you are in another country, is the study/therapy/treatment approved by that country’s medical regulatory agency?
3. What are the pre-clinical safety and efficacy data supporting the use of the proposed cells?
4. Is the study/trial evidence-based?
5. Do you have concerns about the source of the tissue?
6. What is being claimed – such as: better control of symptoms or a cure or…?
7. Is the study being guided by input from experts in the field?
FDA INFORMATION ABOUT REGENERATIVE MEDICINE THERAPIES:
Framework for the Regulation of Regenerative Medicine Products
The FDA has also published two final guidance documents intended to aid in the effort to bring innovative, safe, and effective products to patients as efficiently as possible:
Expedited Programs for Regenerative Medicine Therapies for Serious Conditions
Evaluation of Devices Used with Regenerative Medicine Advanced Therapies
The FDA has offered all operating stem cell clinics an opportunity to file for (FDA) approval based on the data garnered from their “treatments” or “trials”.
Many stem cell clinical trials and therapies operate under a “regulatory grey-area” due to the products being originate from the patient and have undergone minimal manipulation before being “re-introduced” back into the same patient.
In consideration of the possibility that some of the approximated 1,000 clinics in the US may have discovered something of benefit, the FDA has offered an opportunity for these clinics to file for an exception. This offer, now about 2 years old, expires May 31, 2021. To date, not one clinic has filed for an exception.
FDA INFO ABOUT “BAD ACTOR” STEM CELL CLINICS:
FDA Warns About Stem Cell Therapies
Canada case highlights possible long-term risks of experimental stem cell therapies
ARTICLES ABOUT “BAD ACTOR” STEM CELL CLINICS:
FDA: consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes
REFERENCES:
Note: The 7 questions are based on the GForce-PD questions.
https://www.sciencedaily.com/releases/2016/03/160329113350.html
http://www.nature.com/articles/npjparkd201517
Bratt-Leal, Andrés M., and Jeanne F. Loring. “Stem Cells for Parkinson’s Disease.” Translational Neuroscience. Springer US, 2016. 187-201
