Stem Cells 101
What are Stem Cells?
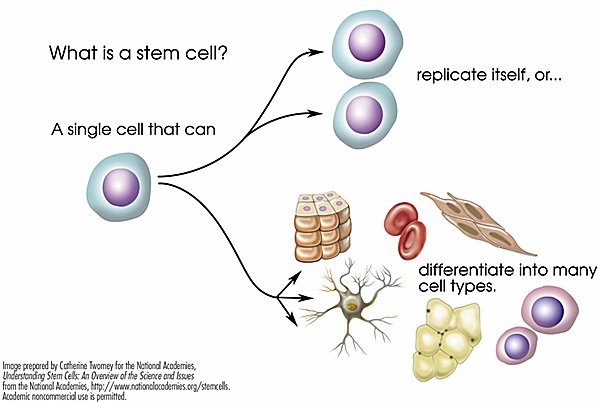
Stem Cells Defined:
- A stem cell is a cell with the unique ability to develop into specialized cell types in the body.
- Stem cells have the ability to self-renew and to recreate functional tissues.
- Stem cells can be used to replace cells and tissues that have been damaged or lost to disease.
- All stem cells are defined by two properties:
- They can make copies of themselves.
- They can become two or more other cell types.
Types of Stem Cells
- There are many different types of stem cells (e.g., bone marrow stem cells, embryonic stem cells, adult stem cells, umbilical cord stem cells, mesenchymal stem cells, etc.)
- What differentiates one stem cell type from another is how many cell types they can become.
- Based on this feature, we can categorize all stem cell types as either multipotent or pluripotent.
Multipotent Stem Cells
Mulitpotent stem cells (MSCs)
- Can self-renew and become at least two different cell types
- Cannot become all cell types in the body
- Senescent (will eventually die)
- Multipotent = “many powers”
- Cannot become dopaminergic neurons (the cells missing in Parkinson’s disease)
- Tissue specific stem cells.
- Limited abilities to become other cell types
- Exist in some but not all of our organs
Hematopoietic stem cells (Tissue speicific stem cells)
- Umbilical cord stem cells – a type of hematopoietic stem cells
- Found in bone marrow
- Can generate cell types for the specific tissue or organ in which they live.
- Can make all types of blood cells – only.
- Can not make cells of the brain.
- More prevalent in fetal tissue than adult tissue.
Mesenchymal stem cells
- Originate in connective tissue that surrounds other tissues and organs.
- Can be isolated from many tissues including
- Bone marrow,
- Fat (adipose tissue)
- Umbilical cords
- Have narrow differentiation abilities
- Capable of making three cells types: Bone, cartilage, and fat cells.
- Some – but not all – may transiently reduce tissue inflammation.
- MSCs have been tested in many clinical trials but, so far, have not resulted in: no significant results in diverse diseases such as: stroke and heart disease
Mesenchymal stem cells
- Derived from body fat
- Often used in unregulated clinics claiming to treat a large range of diseases
Neural Stem Cells
- Cells from some parts of the brain
- Limited ability to replace some nerve cells & supportive cells
Pluripotent Stem Cells
Pluripotent Stem Cells :
- Ability to self-renew and become all cell types in the body.
- Pluripotent = “Very many powers”.
- Immortal (Never die).
- Do not exist in the body. Only briefly present in very early embryos.
- Can be generated in the lab.

Human Embryonic Stem Cells
- Pluripotent stem cells that can become any cell in the body.
- Generated from 5 day old embryos that are produced in a culture dish by combining an egg & sperm.
- Discarded because they are unnecessary or unsuitable for transferring to uterus for pregnancy. (“In vitro fertilization”)
Parthenogenetic stem cells
- Type of pluripotent stem cell
- Created by calcium activation of oocytes.
Induced Pluripotent Stem Cells (iPSCs)
- Generated in lab from adult somatic (body) cells using a method called “reprogramming”
- Generally, skin cells
- Type of pluripotent stem cell
- Behaves exactly the same as embryonic stem cells.
- Can become any cell in the body.
Stem Cells & Their Characteristics

Induced pluripotent stem cells (iPSCs)
- iPSCs are a type of pluripotent stem cells that is particularly amenable to therapeutic uses.
- One of the most important features of iPSCs is that they can be made from any living person so they can be immune-matched (or DNA-matched) to any patient which will obviate the need for immune suppression when used in cell therapy. Immune matched cells are referred to as “autologous,” while unmatched cells are “allogeneic.”
- Additionally, since iPSCs can be made from any person, they can be used to model any disease.
How are iPSCs made?
- Typically, skin cells (fibroblasts) or blood cells from adults are converted into iPSCs through a process referred to as “reprogramming.”
- The reprogramming process is typically mediated by viruses or mRNAs that force the cell to produce proteins involved in pluripotency.
- Dr. Shinya Yamanaka received the Nobel Prize in Medicine in 2012 for developing this process.

How do stem cells "become" other cell types?
The process of “differentiation”
- Differentiation is the process by which cells change from relatively generalized cells to specialized kinds of cells during embryonic development.
- In the laboratory this can be accomplished by exposing stem cells to cues (e.g. hormones, growth factors, chemicals, substrates, small molecules, etc.) that recapitulate the stimuli they would normally be exposed to in the developing embryo. With time in the culture dish, stem cells exposed to the proper differentiation cues will eventually mature into the desired cell type and function in a way similar to its comparable cell type that developed normally in the body.
- Some cell types are hard to make, some are easy. With time and effort, the combination of factors required to make any cell type can be discovered.

The promise of regenerative medicine
Treating Parkinson’s disease and beyond…..
- Many diseases are caused by a cell type that ceases to function or does not function properly (classic examples: dopamine neurons in Parkinson’s disease, islet cells in diabetes, retinal pigment epithelial cells in macular degeneration, etc)
- Pluripotent stem cells can be differentiated into any cell type in the body
- Using pluripotent stem cells we can make more of any missing or defective cell type in the laboratory and transplant it into the patient
- If we use patient-specific iPSCs, then we do not need immune suppression which carries a number of its own problems

- The figure (right) illustrates the promise of regenerative medicine in treating Parkinson’s disease using an autologous dopamine neuron replacement strategy.
- Skin fibroblasts are isolated from the patient and then the cells are reprogrammed to make iPSCs.
- The iPSCs are differentiated into dopamine neurons.
- These DNA-matched dopamine neurons are then transplanted back into the patient where they will produce more dopamine and ostensibly relieve Parkinson’s symptoms.
Stem cells in the clinic
- There are many notable stem cell-based, FDA-approved clinical trials and therapies that exist or have taken place in the US. The most important is the bone marrow transplant which uses hematopoietic stem cells (a multipotent stem cell) to repopulate the blood in patients with cancer, blood disorders or immune diseases. This therapy was started in the 1950s and currently is the only FDA-approved stem cell-based therapy.
- The first clinical trial involving pluripotent stem cells began in 2008 and sought to treat spinal cord injury. The trial was initially sponsored by a company called Geron which used an hESC-derived oligodendrocyte progenitor cell, a cell type that supports the motor neurons injured in spinal cord injury.
- More recently, several clinical trials using stem cells for the treatment of eye diseases including macular degeneration, Stargardt’s Macular Dystrophy and Retinitis Pigmentosa, have taken place. Many of these studies have used a stem cell-derived retinal pigment epithelial cell progenitor which is a cell type that supports the rod and cone cells necessary for vision. Of note, many of the patients in these trials have shown significant increases in visual acuity in the transplanted eye suggesting that this may be a novel breakthrough treatment for these eye diseases. In August of 2020, a clinical trial for Macular Degeneration using autologous iPSC-based therapy began recruiting patients.
- A stem cell-based clinical trial using to treat Type 1 Diabetes was initiated by Viacyte in 2014. This trial uses a unique encapsulation system to protect the hESC-derived pancreatic precursor cells from immune attack.
- We are eagerly awaiting Aspen Neuroscience’s autologous iPSC-derived dopamine neuron replacement clinical trial!
FDA-approved stem cell-based therapies and clinical trials in the US

Stem cells: today and tomorrow
Are there other uses for stem cells?
- We’ve already discussed how stem cells can be used for cell therapy but stem cells have many other uses, some of which will be important for Parkinson’s and other neurological diseases.
- Disease Modeling – Stem cells, and in particular iPSCs, made from someone with a particular disease show hallmarks of that disease when the cells are differentiated into the cell type affected by the disease. For example, it is well-established that people with Parkinson’s disease show an abnormal accumulation of α-synuclein in their brain. iPSCs derived from Parkinson’s patients that are differentiated to dopaminergic neurons also produce abnormally large amounts of α-synuclein. What this means is that stem cells can be used as an infinite source of diseased cells that accurately reproduce the disease in a dish. From there, these cells can be used to screen for drugs that correct problems associated with that particular disease. Currently, iPSCs and hESCs are widely used in drug screening to find treatments for Parkinson’s and other neurological diseases.
- Cytotoxicity testing – Once a new drug has been developed, researchers must determine whether it has unwanted cytotoxic side effects. Of particular concern are neurotoxicity, cardiotoxicity and hepatotoxicity. Finding appropriate cell types to use for cytotoxicity testing that resemble normal neurons, cardiomyocytes and liver cells is difficult. Since one of the defining features of pluripotent stem cells is that they can be differentiated into any cell type in the body, hPSCs represent an unlimited source of cells that can generate normal neurons, cardiomyocytes, and liver cells that can then be used for cytotoxicity testing.
- Saving endangered species – Species are becoming extinct at an alarming rate. Many different strategies have been employed to save them with varying effectiveness. Pluripotent stem cells can become any cell type in the body including eggs and sperm. A new approach to saving endangered species is making iPSCs from these animals and then differentiating them into eggs and sperm. From there, in vitro fertilization can be used to generate new embryos from that endangered species which can be implanted in the uterus of a similarly sized species. Ultimately, this approach may be used to create new individuals of a particular endangered species. This idea was first envisioned by Dr. Jeanne Loring and is currently being developed with the Northern White Rhino at the San Diego Zoo Safari Park.
- Cell-based Meat – Traditional approaches to meat production involve growing animals for a long period of time, feeding them, taking up lots of land, only to eventually slaughter them and send them to market. This negatively impacts our environment and many people are concerned that it promotes animal suffering. An alternative to traditional meat production is cell-based meat where meat is produced by culturing animal cells in the laboratory. Many companies are currently developing cell-based beef, chicken, pork and fish. Some of these companies are starting with iPSCs from cow, pigs, chicken or fish and then differentiating those cells into the cells that are present in a meat fillet including muscle, fat and connective tissue. From there, the cells can be molded or bio-printed into fillets. While not yet commercially available, these companies may be major meat suppliers in the future.
- People continue to come up with new ways to use stem cells. The future uses of stem cells may surprise us all!

References:
- https://www.sciencedaily.com/releases/2016/03/160329113350.html
- http://www.nature.com/articles/npjparkd201517
- http://www.journalofparkinsonsdisease.com/are-stem-cell-therapies-parkinson%E2%80%99s-disease-ready-clinical-trials Note: The 5 questions are based on the GForce-PD questions.
- Bratt-Leal, Andrés M., and Jeanne F. Loring. “Stem Cells for Parkinson’s Disease.” Translational Neuroscience. Springer US, 2016. 187-201.
- http://www.nature.com/nrneurol/journal/v11/n9/full/nrneurol.2015.123.html
Additional Resources:
- Stem Cell & Regenerative Medicine Glossary

